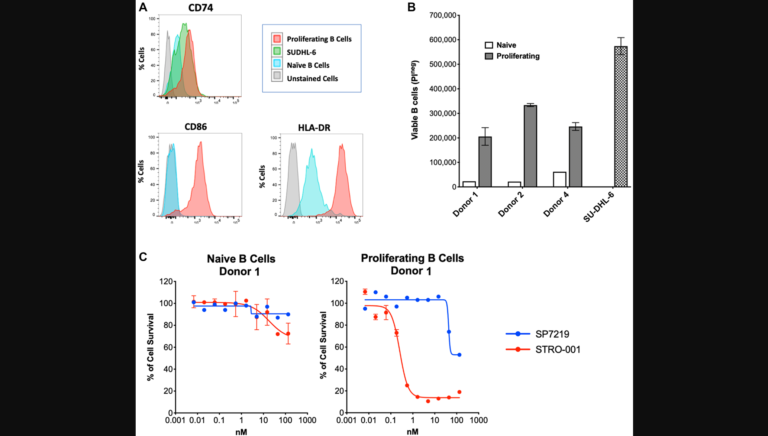
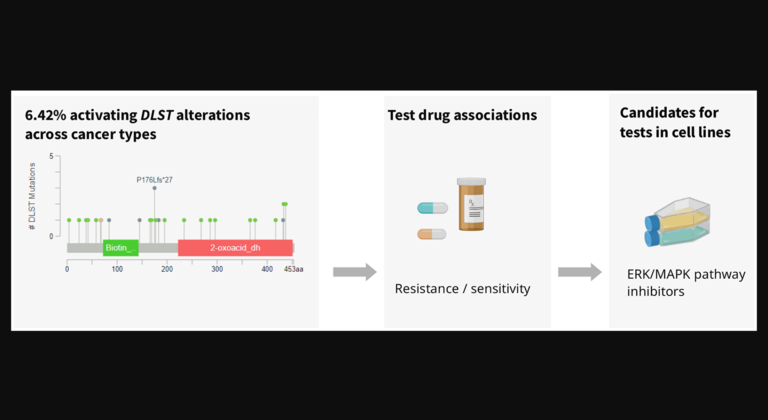
“In this study, we present findings for a protective role of RORα in a mouse laser-induced CNV model of neovascular AMD.”
BUFFALO, NY- January 18, 2023 – A new research paper was published in Aging (listed as “Aging (Albany NY)” by MEDLINE/PubMed and “Aging-US” by Web of Science) Volume 15, Issue 1, entitled, “Genetic deficiency and pharmacological modulation of RORα regulate laser-induced choroidal neovascularization.”
Choroidal neovascularization (CNV) causes acute vision loss in neovascular age-related macular degeneration (AMD). Genetic variations of the nuclear receptor RAR-related orphan receptor alpha (RORα) have been linked with neovascular AMD, yet its specific role in pathological CNV development is not entirely clear.
In this new study, researchers Chi-Hsiu Liu, Felix Yemanyi, Kiran Bora, Neetu Kushwah, Alexandra K. Blomfield, Theodore M. Kamenecka, John Paul SanGiovanni, Ye Sun, Laura A. Solt, and Jing Chen from Harvard Medical School, UF Scripps Biomedical Research and University of Arizona showed that Rora was highly expressed in the mouse choroid compared with the retina, and genetic loss of RORα in Staggerer mice (Rorasg/sg) led to increased expression levels of Vegfr2 and Tnfa in the choroid and retinal pigment epithelium (RPE) complex.
“Here, we investigated whether RORα regulates CNV using a mouse model of laser-induced CNV, mimicking the neovascular features of wet AMD. We found that expression of RORα was enriched in the mouse choroid/RPE complex and upregulated in laser-induced CNV.”
In a mouse model of laser-induced CNV, RORα expression was highly increased in the choroidal/RPE complex post-laser, and loss of RORα in Rorasg/sg eyes significantly worsened CNV with increased lesion size and vascular leakage, associated with increased levels of VEGFR2 and TNFα proteins. Pharmacological inhibition of RORα also worsened CNV. In addition, both genetic deficiency and inhibition of RORα substantially increased vascular growth in isolated mouse choroidal explants ex vivo. RORα inhibition also promoted angiogenic function of human choroidal endothelial cell culture.
“Together, our results suggest that RORα negatively regulates pathological CNV development in part by modulating angiogenic response of the choroidal endothelium and inflammatory environment in the choroid/RPE complex.”
DOI: https://doi.org/10.18632/aging.204480
Corresponding Author: Jing Chen
Corresponding Email: jing.chen@childrens.harvard.edu
Keywords: age-related macular degeneration, angiogenesis, choroidal neovascularization, inflammation, nuclear receptors, RORα, VEGFR2, TNFα
Sign up for free Altmetric alerts about this article: https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.204480
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
About Aging-US:
Launched in 2009, Aging (Aging-US) publishes papers of general interest and biological significance in all fields of aging research and age-related diseases, including cancer—and now, with a special focus on COVID-19 vulnerability as an age-dependent syndrome. Topics in Aging go beyond traditional gerontology, including, but not limited to, cellular and molecular biology, human age-related diseases, pathology in model organisms, signal transduction pathways (e.g., p53, sirtuins, and PI-3K/AKT/mTOR, among others), and approaches to modulating these signaling pathways.
Please visit our website at www.Aging-US.com and connect with us:
- SoundCloud – https://soundcloud.com/Aging-Us
- Facebook – https://www.facebook.com/AgingUS/
- Twitter – https://twitter.com/AgingJrnl
- Instagram – https://www.instagram.com/agingjrnl/
- YouTube – https://www.youtube.com/agingus
- LinkedIn – https://www.linkedin.com/company/aging/
- Reddit – https://www.reddit.com/user/AgingUS
- Pinterest – https://www.pinterest.com/AgingUS/
For media inquiries, please contact media@impactjournals.com.